Medicines Agency agreed to vaccinate 12-year-children
12:47 | 26.10.2021 Category: Social
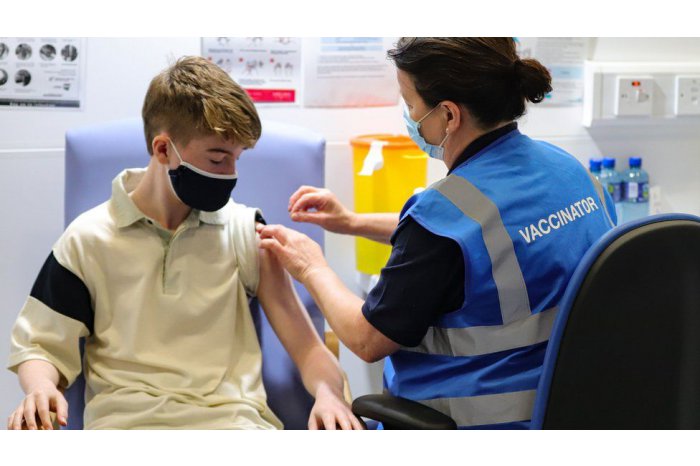
Chisinau, 26 October /MOLDPRES/- The Agency for Medicines and Medical Devices / AMDM / announced today that it agreed to vaccinate children aged 12 years with Pfizer.
According to AMDM data, on 25 October the Medicines Commission of the Medicines and Medical Devices Agency approved the vaccination of 12-year-olds with Comirnaty serum made by Pfizer-BioNTech.
The decision will be submitted for approval in accordance with the official information of the World Health Organization on COVID-19 vaccines. Also, with the approval of the commission, for the Pfizer Comirnaty vaccine, the shelf life of the product, ie the frozen vial, was changed. In the current version it is 6 months, the 9 month version is proposed for approval.
Also yesterday, the Medicines Commission authorized 57 pharmaceuticals. Among the new products approved, for the first time, by the Commission are drugs such as: Ozempic - solution for injection for patients with diabetes; Orabloc - a new type of local anesthetic, Salbutamol - antiasthmatic product; Povidon-iodine - antiseptic and disinfectant, Startum powder and solvent for solution for injection - are vitamins \ multivitamins in combination; Oseltamivir Avexima - a new type of antiviral drug product. At the same time, it approved 577 post-authorized amendments for 139 medicines.
By majority vote of the commission members, the authorization procedure in the case of 34 medicinal products was interrupted. This was caused by a number of factors, including the inadequate presentation of the package of documents, and the rejection of the objections raised by the Agency's experts in the terms set by law.
Photo: bbc.com